
Topic: Diffusion
Part One: Teacher Notes
Sate and National Standards:
Missouri Department of Elementary and Secondary Education:
Strand 3: Characteristics and Interactions of Living Organism
Section 2) Living organisms carry out life processes in order to survive:
Explain the significance of semi-permeability to the transport of molecules across cellular membranes.
Relate the role of diffusion to the movement of molecules across a semi-permeable membrane.
National Science Education Standard:
Science Content Standards: 9-12, Science as Inquiry:
Every cell is surrounded by a membrane that separates it from the outside world.
Concept:
The concept being addressed in this lesson is diffusion. Diffusion is an example of passive transport whereby ions or molecules move down their concentration gradient. During diffusion, molecules in an area of relatively high concentration move to an area of relatively low concentration. There fore, this process does not require energy.
Exploration:
The activity that has been picked for the exploration part is a very simple activity. The students will be put in groups of 3-4 by me (the teacher). The direction for the activity will be given on a separate sheet of paper. In this activity, the students will put a few drops of food coloring in a beaker filled with water and will answer a few questions regarding their observation. Click here to see what this experiment should look like. http://video.google.com/videoplay?docid=-5242394503257451479&q=diffusion&hl=en
For this part, the students will be required to present their hypothesis to the classroom. After all students have presented their hypothesis, “the food coloring activity” worksheet will be collected and graded by me.
Concept Introduction:
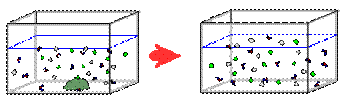
In the experiment the food coloring represents molecules of high concentration. When mixed with the water, which has a lower concentration of food coloring, the molecules in the food coloring diffuse (spread out) to fill the entire container evenly, which is called equilibrium.
The molecules of food coloring are in constant motion, as are all molecules. Many of these molecules move away from the concentration of food coloring, just because of their random direction. Now, since there are more food coloring molecules moving out of the concentrated area than there are moving into it, the food coloring spreads out until it fills the entire container of water.
This activity is a great way to introduce diffusion to the students. By doing the activity first, some might be able to know what is happening, but just can’t put it in its scientific terms. The concept introduction will introduce some new concept that will help them understand what is happening and how it’s happening.
Concept Introduction Notes:
The cell membrane regulates what enters and leaves the cell and also provides protection and support.
The cell takes in food and water and eliminates wastes through the cell membrane.
The core of nearly all cell membranes is a double-layered sheet called a lipid bilayer.
The bilayer gives cell membranes a tough, flexible structure that forms a strong barrier between the cell and its surroundings.
Diffusion is a very basic principle of liquids and gasses. When diffusion occurs, molecules in an area of relatively high concentration move to an area of relatively low concentration.
The concentration of a solution is the mass of solute in a given volume of solution, or mass/volume.
Diffusion causes many substances to move across a cell membrane but does not require the cell to use energy.
Application:
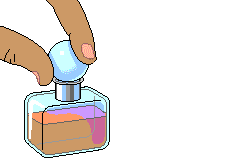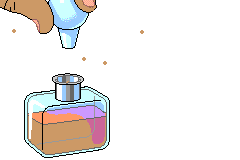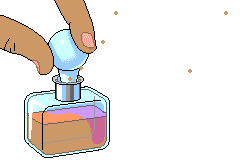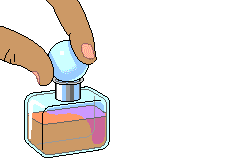
For the application part, I have provided two different activities to help the students understand the concept of diffusion. The first concept application is very simple. In this section of the lesson, I will present a perfume bottle in front of the classroom and will simply open the bottle. The students are to let me know when they smell the perfume. Since the students are in different rows and are in different distance from the perfume, each row should smell the perfume at a different time.
When a bottle of perfume is opened at the front of a room, within minutes people further and further from the source can smell the perfume. This defines diffusion: Diffusion is the net movement of molecules down their concentration gradient. The students are responsible for answering the questions given individually and we will discuss the answers as a class when all the students have had time to answer the question.
The second concept application involves the action of salt being dissolved in water. In this experiment, the students will pour a table spoon of salt into a beaker of water and watch it dissolve. They will be working in groups of 3-4 and will have to respond to the questions given.
Explanation of what is happening: Above the pile of crystals, a dense concentration of ions (Na+ and Cl-) begins to form. The further away from the pile the fewer the number of ions of Na+ and Cl- that exist, therefore, producing a decreasing concentration gradient.

Diffusion acts to further spread out the Na+ and Cl- ions. Note also that the water molecules are also diffusing from an area of higher concentration of water (away from the pile of salt crystals) to an area of lower concentration of water (near the pile of salt crystals). The ions Na+ and Cl- ions are moving down their concentration gradients, which is away from the crystals.
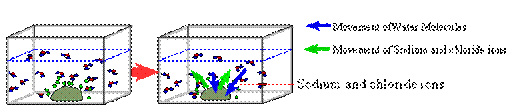 Eventually
when all the salt has dissolved in the water and diffusion has reached
equilibrium, a homogeneous solution of NaCl in water will form salt water.
Eventually
when all the salt has dissolved in the water and diffusion has reached
equilibrium, a homogeneous solution of NaCl in water will form salt water.
Material List:
For the exploration part, the students will need water, a beaker, and food coloring per group. For the application part, the students will be observing only and I will need a perfume bottle.
History of Concepts:
In 1829, T. Graham studied diffusion of gases from a closed vessel through a small tube into the surrounding air.
In 1855, A. Fick formulated his mathematical statement of the law of diffusion by analogy with Fourier’s law of heat conduction.
Related Websites:
http://www.phschool.com/science/biology_place/labbench/lab1/concepts.html
http://hyperphysics.phy-astr.gsu.edu/Hbase/kinetic/diffud.html
http://www.geocities.com/piratord/Difus.html
Miller, levine. Biology. Pearson Education, Inc., New Jersey:2002.
References:
http://www.phschool.com/science/biology_place/labbench/lab1/concepts.html
Miller, levine. Biology. Pearson Education, Inc., New Jersey:2002.
http://www.geocities.com/piratord/Difus.html
Part Two: Students Pages
Exploration: The Food Coloring Activity:
For this experiment each group will need:
Water
A beaker
Food coloring
Procedure:
1. Fill the beaker 3/4 of the way with tap water.
2. Put a few drops of food coloring into the jar of water.
Please answer the following questions:
1. What happens as you make your drops?
2. What happens with the food coloring after a few minutes has passed?
3. What happens to the water in the beaker after a few minutes has passed?
4. What do you think is happening?
5. What can you conclude from your observation?
6. State your hypothesis and be ready to present your hypothesis and explain why you have come up with such hypothesis.
Concept Application : Perfume Bottle
For this activity I need all of you students to sit at your assign seat and not move.
I will open a perfume bottle in front of the room, where all of you can see it.
All you guys have to do is to raise your hand the second you smell the perfume from where you are seated.
After all of you have smelled the perfume, answer the following questions.
1. Who smelled the perfume first? Why?
2. Who smelled the perfume last? Why?
3. Can you explain what is happening in the classroom?
4. What is causing the perfume to move around the classroom, so you can smell it?
For this experiment each group will need:
A beaker
Water
A table spoon of salt crystals (NaCl)
Procedure:
1. Fill up the beaker ¾ of the way with water.
2. Add a table spoon of salt crystals to the water. (DO NOT MIX!!!!!!!!!!)
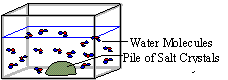
Please answer the following questions:
1. Observe and write down what is happening in the beaker as time passes by.
2. Can this process be called diffusion? Why?
Back to the Learning Cycle Unit